With the Fourth of July just around the corner, it is that time of the year when the skies across the United States light up with vibrant and spectacular fireworks. As we all prepare to celebrate Independence Day with friends and family, let’s take a moment to appreciate the science behind the breathtaking fireworks that have become a hallmark of this holiday.
The Beauty in Chemistry
Fireworks are not just about bright lights and loud bangs. There is intricate chemistry involved in their creation. At IXRF Systems, we are fascinated by the elemental composition of things, and fireworks are no exception!

Figure 1. A Fourth of July firework celebration and the associated chemistry identifying the different elemental compounds that provide their vibrant colors.
The Core Elements
Several elements play a vital role in the creation of fireworks. When an element burns, it produces a particular color. By combining various compounds, firework manufacturers can create a vast palette of colors and effects.
- Strontium & Lithium Compounds – If you’re seeing red in the sky, it’s probably thanks to strontium or lithium salts. Strontium carbonate, for example, is often used to produce deep reds.
- Calcium Salts – Calcium salts create an orange color. One of the common salts used is calcium chloride.
- Sodium Compounds – Sodium compounds produce a yellow flame. A familiar example is sodium nitrate.
- Barium Salts – Green is the color of barium. Barium chloride is the salt often used to create stunning green colors.
- Copper Compounds – When you see blue in the sky, it’s the result of copper compounds being heated. Copper chloride is one of the commonly used compounds.
- Strontium & Copper Compounds and Potassium & Rubidium Compounds – These elements are used to create purple colors. Potassium nitrate and rubidium compounds are common choices.
- Zirconium, Titanium, & Magnesium – These metals are used to produce bright white sparks. They are highly reactive and create brilliant, intense bursts of light.
Elemental Analysis with IXRF Systems
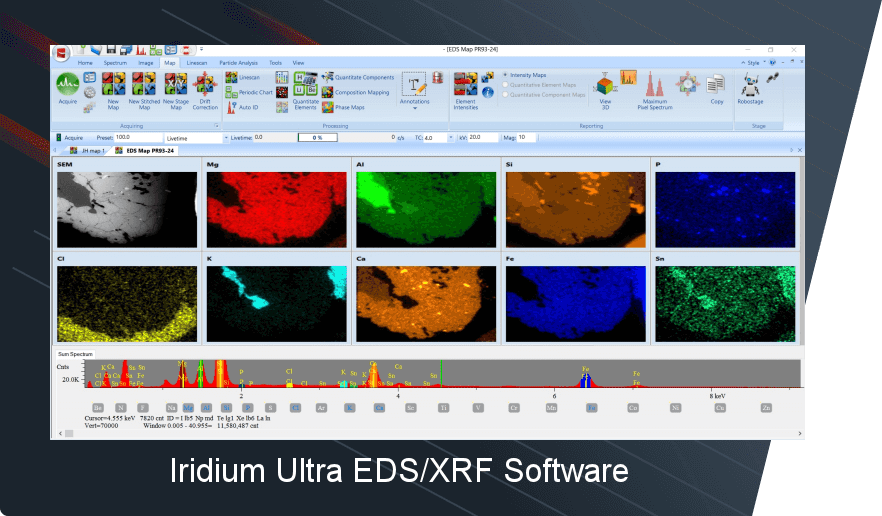
At IXRF Systems, we’re passionate about elemental analysis. Our state-of-the-art X-ray fluorescence (XRF) and Micro-XRF instruments are utilized in various fields to identify and analyze the elemental composition of materials.
In the context of fireworks, XRF technology can be incredibly useful. For example, by utilizing XRF, manufacturers can verify the presence and concentration of particular elements in their fireworks. This ensures that they meet quality standards and create the desired colors and effects.
A Salute to Tradition and Science
As you watch the fireworks light up the sky this Fourth of July, remember the chemistry that makes it possible. The vibrant colors that dazzle us are the result of meticulously combined elements that have been refined through centuries of experimentation and tradition.
From all of us at IXRF Systems, we wish you a safe and happy Independence Day!
Take the Next Step in Elemental Analysis
Are you as intrigued by the science behind fireworks as we are? IXRF Systems offers the tools you need to explore the elemental composition of materials, including the ones used in fireworks! Whether you are in research, quality control, or education, our advanced X-ray fluorescence (XRF) and Micro-XRF instruments are designed to help you achieve new levels of understanding and precision in your work.
This Fourth of July, let’s celebrate the innovation that comes with understanding the elemental compositions around us. Reach out to us and discover how IXRF Systems can revolutionize your approach to elemental analysis.
IXRF Systems is a leading provider of X-ray fluorescence instrumentation. With our advanced analytical solutions, we are committed to supporting research, quality control, and educational endeavors across various industries.